/ Additive manufacturing properties
Bulk material properties
Implantable-grade titanium (Ti) and Ti alloy (Ti6Al4V) are used in the additive manufacturing process. Their chemical and mechanical properties comply with ISO and ASTM Standards.
Implantable-grade Ti and Ti alloys are used in Lincotek Medical’s additive manufacturing processes
| Ti6Al4V EBM |
Ti6Al4V Laser |
ASTM F136 |
|
| E (MPa) | 118 ± 5 | 111 ± 1 | N.Ap. (104 ± 2*) |
| UTS (MPa) | 914 ± 10 | 1073 ± 4 | > 860 |
| ¨L (%) | 13,1 ± 0,4 | 12,0 ± 0,2 | > 10 |
| Alternate Bending Fatigue limit @2*10^6 cycles (MPa) | 441 ± 42 | 440 ± 53 | N.Ap. (445 ± 7*) |
Ti and Ti alloys used in our additive manufacturing processes comply with ISO and ASTM standards
Porous structure properties
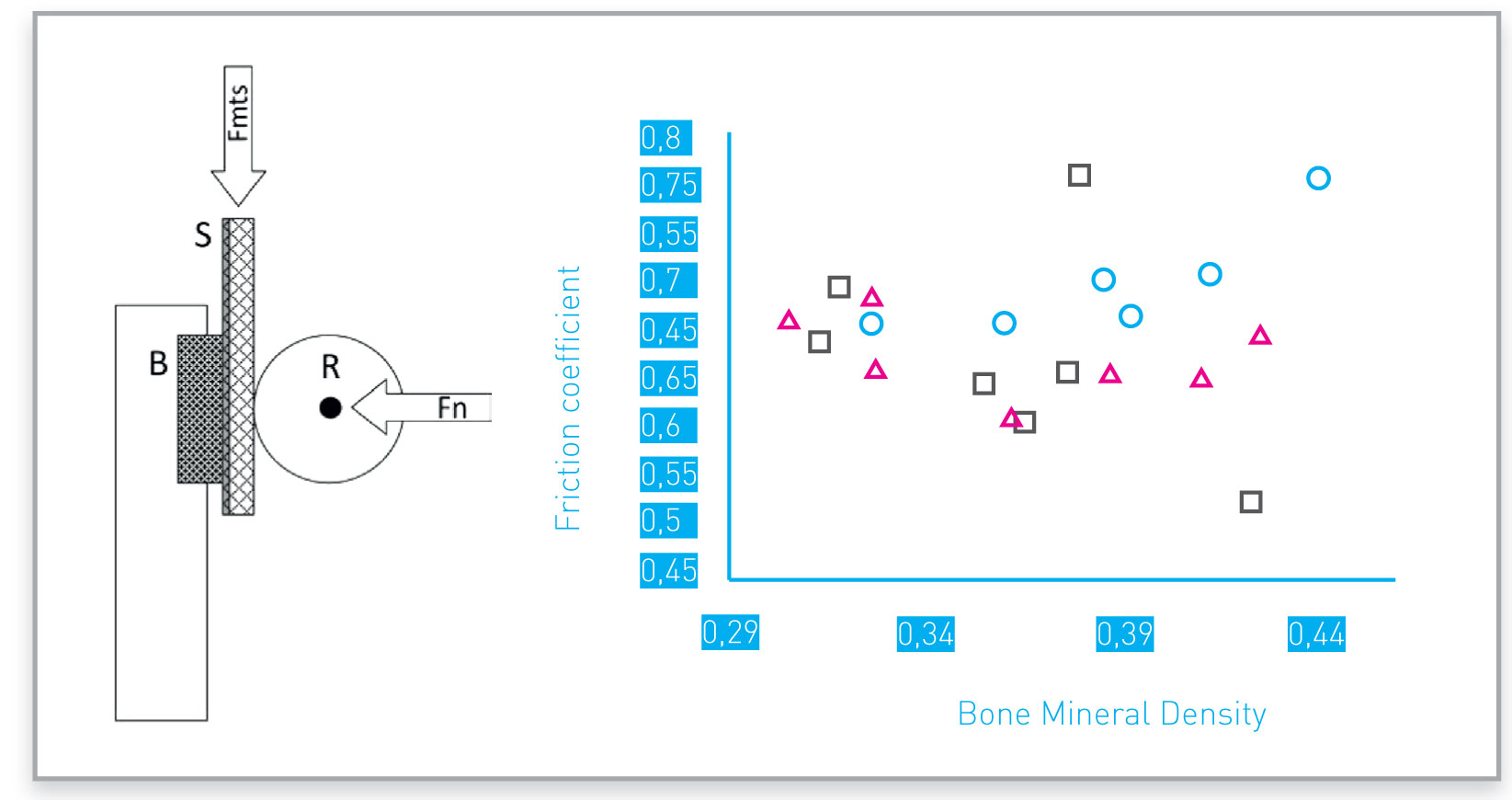
Example of friction coefficients obtained with different porous structures.
Symbols identify four different porous structures tested
With additive manufacturing, 3D metal networks can be CAD-designed and built up together with devices’ solid parts.
We offer design and manufacturing of porous engineered porous structures.
Porous surface can be designed and manufactured to achieve desired friction against bone: one example is reported in the picture.
Additively manufactured metal porous structures can be designed and manufactured to achieve desired adhesion and cohesion strengths, in full agreement with applicable standards. One example is shown here.
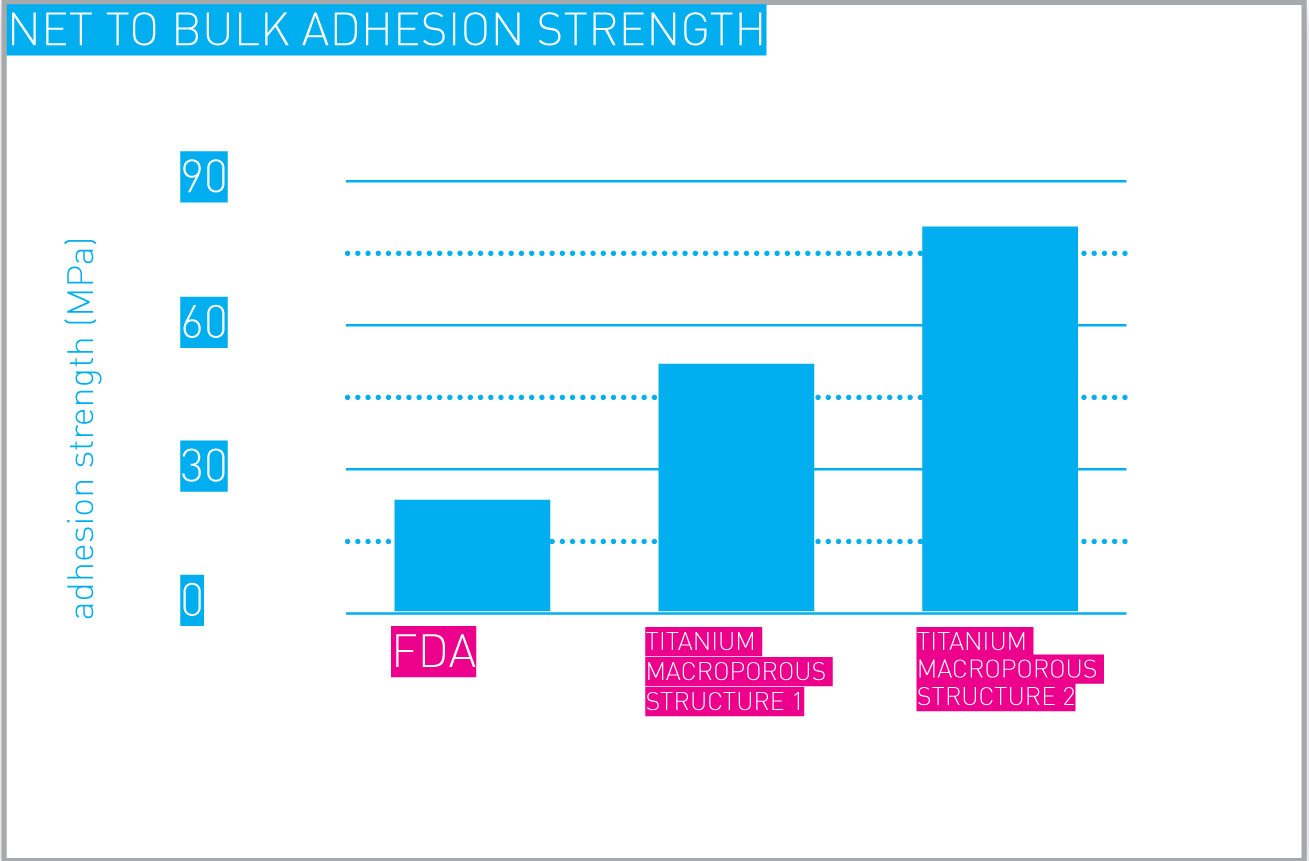
Net to bulk adhesion strength.
Values shown are relative to a couple of porous structures designed and manufactured for exemplification purposes.
Biological porous structure characterization
In vitro tests have assessed cell viability and proliferation in additive manufactured Ti networks.
For properly-designed and manufactured porous structures in vivo tests have shown:
Contact us for more details or download our brochure which includes further information
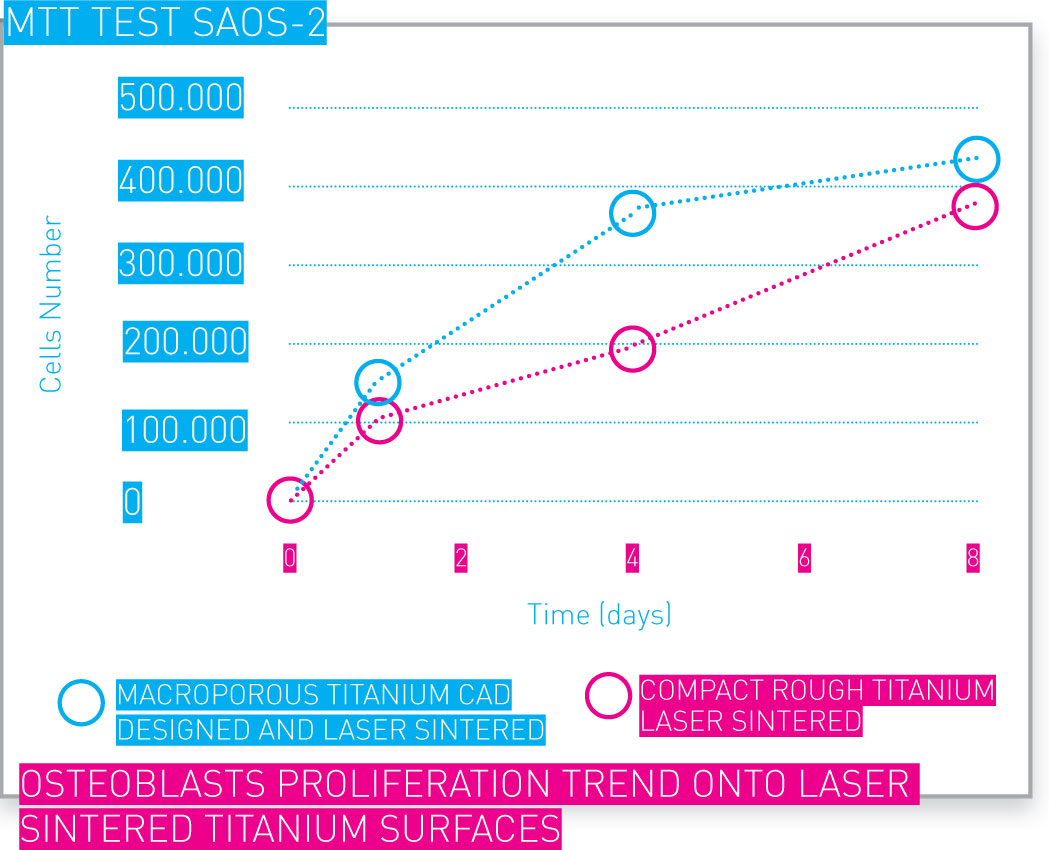
Osteoblasts proliferation trend onto laser sintered Ti surfaces
